MARKTECH |
|||||||||||||||||||||||||||
|
Section 1 How/When it Started Thurston County Animal Shelter in Washington was the first shelter to begin using the microchips. They began the chip implantation program in 1991. By scanning the area of the microchip insertion, shelters can tell immediately who the owner is of the stray pet they are scanning. The chip sends back a reference number which can be checked in several national databases. The ID number reveals who the pet’s owner is, where the owner lives, the vet performing the service, and the shelter where the animal was implanted with the microchip device. The easiness of identification allows for immediate return of the animal to its owner, eliminating the need for a standard 2 day holding period. Using RFIDs reduces the shelter expenses for holding stray pets.
Section 2 How it Works Surrounding this assembly there is an anti-migration cap to impede migration of the implants within the body of the animal. The patented BioBond anti-migration cap is a porous polypropylene polymer sheath attached to RFID microchip implants. The use of the BioBond cap results in increased retention by promoting the development of fibroblasts and collagen fibers around the implant, thus inhibiting movement of the RFID within the tissue where the device was placed. Each chip is individually inscribed with a unique identification number during the manufacturing process to ensure that no two RFIDs have the same code number. Each microchip is programmed to store an alphanumeric identification code. The inscribed number identifies the chip by its manufacturer, while the programmed alphanumeric code identifies the animal or human.
Section 3 Microchip Manufacturers There are three major microchip systems in the US.
Section 4 Size of the RFID Device
The microchip assembly has the size of "a grain
of rice". It varies between 1 to 2 cm in length, and about
1/4 cm in diameter (less
than one inch in length and 1/8 of an inch in
width) By reducing the size of the components
of the RFID it has become possible to insert this device inside the animal or
person carrying the implant. The benefits for wildlife is that it cannot
be lost. The benefits for future human users is that it will not be able
to be disposed quickly by kidnappers as the case with bracelets will be.
Section 5 Syringes The syringes that are actually in use for animal implantation in veterinary offices use either liquid or air to push the implant into the site under the skin.
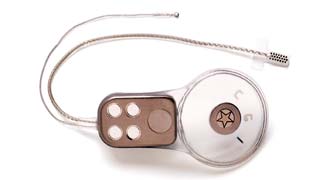
Section 6 Readers To be able to read information from the microchip, there are already hand held readers and pocket readers.  

As a scanner is passed over the chip, it sends out low level radio waves which penetrate the inert glass seal and strike the antenna. This creates a small amount of electric current which is stored by the capacitor. The capacitor sends the current to the microchip, which uses the current to access its stored alpha- numeric code. The microchip then sends the code to the antenna, where it will be picked up and read by the scanner. An LCD on the scanning device will then show the ID code.
Section 7 Human Implantable Devices To make it palatable for the consumer, the idea of implants has gradually developed. In the same way, the mark of the beast will evolve from a bracelet type device to an implanted device in the hand, and finally to an implanted device in the forehead/brain. Here are some examples of current implanted devices. 1. Cardiovascular pacers 2. Brainstem implants
Medtronic Kappa® Pulse nucleus 24 Auditory Brainstem Implant (ABI) 3. MEMS (Micro Electro-Mechanical System) The micro-electro mechanical systems device (MEMS) is an implantable micro-sensor that can send data to a hand-held receiver outside the body, alerting doctors to a potential medical crisis, without using any wires or batteries. Doctors can check the condition of a patient's heart by holding the receiver near the patient. Patients can even monitor their condition at home. Initial data on the MEMS was presented in January of 2002 at the 14th annual International Symposium on Endovascular Therapy in Miami Beach, Florida. The MEMS can monitor blood pressure levels in patients with heart failure or with abdominal aortic aneurysm, an abnormal widening of the aorta. If it works, it could provide doctors with an easier way to catch serious problems. This device is implanted directly in the area under study, such as in an aortic aneurism. The reader device outside the body is the monitor. By using more implantable medical
devices the public becomes desensitized to the idea of more sophisticated
technology being implanted inside the body.
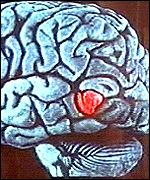
The Brain Pacemaker on the right stimulates the brain and helps prevent seizures. This type of pacemaker works by stimulating the vagus nerve. It consists of a pulse transmitter, which looks like a pocket watch, along with proper wiring. It is implanted in the patient's chest and then connected by wire to the left vagus nerve, which runs down the neck and connects the brain stem with the heart, lung and stomach. The pacemaker periodically gives electrical stimulation to the brain to avoid the onset of seizures. They now want to study this type of implant and examine its use on depressed patients. About 20% of depressive disorders do not respond to either antidepressants or psychotherapies. Scientists plan to use the pacemaker in this group of patients.
Another method being considered to treat depression is Transcrannial Magnetic Stimulation (TMS). The patient sits down on a chair and has an octagonal coil of between 10 to 15 centimeters diameter placed on their head. The coil then carries a current several times per second and generates rapidly pulsating magnetic fields, which stimulate the left forebrain - an area linked to depression Other types of implants approved by the FDA which are inserted inside the body of the patients are: insulin pumps, drug delivery pumps, pain control systems, and nerve stimulators.
Section 8 RFID/VeriChip in Humans The use of microchips in animals -- now a legal requirement for anyone wanting to ship a cat or dog abroad -- seems fairly harmless. Extending the use to the human population is the next step. An x-ray of a hand with the implant is included so the reader could
compare the human anatomy. The availability of implants for use in the human population of the US is no longer the preserve of fiction. One US-based company, Digital Angel, a subsidiary of Applied Digital Solutions (ADS), has already developed the technology for a tracking implanted device to be used in the human population. The makers dwell on the benevolent uses of the device -- such as allowing doctors to monitor medical conditions. Opposition from religious groups, and necessity of FDA approval has delayed the use of the embedded chips. In December 2001, Digital Angel came out with the VeriChip™. In February 2002 it also applied for the trademarks "get chipped" and "Chipsons" regarding the Florida family, the Jacobs', the first family to be implanted. In June of 2002, ADS was to receive approval for human use of the VeriChip. The chips are being sold in South America where they do not need FDA approval.
Head of Privacy International, Simon Davies, believes chips could be employed as tracking devices. "The pattern for these things is they start as medical uses, then becomes used in the military or in prisons. Then become voluntary, then compulsory," he says. Chips are already required in patients who have hip replacements and other prosthetic devices. The continuation of acts of terrorism in the US, especially after the incidents on September 11, 2001 has removed most opposition to implantation. ADS chief technology officer Keith Bolton said "Right now we have over 2,000 kids who have e-mailed, wanting to have the chip implanted," he said. "They think it's cool."
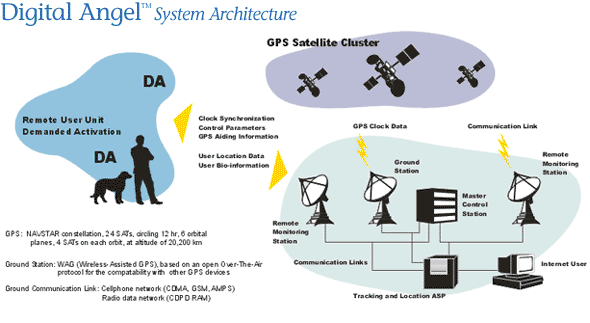
Section 9 Digital Angel Digital Angel systems consist of a unique combination of sensor, location and Web-enabled wireless technologies to provide transmission of critical sensory and biosensory data and location information in real time."
"Digital Angel's pulse sensors are based on infrared radiation naturally emitted from the wearer's own bloodstream. Our engineers are also developing sensors to allow measurement of a great many factors, which operate by utilizing selected wavelengths of light - with no skin breakage whatsoever." "EKG and EEG sensors will be available shortly. So will Digital Angel's solid-state Heat Cells - another unique, proprietary component to our technology - which will power Digital Angel products by utilizing body heat to produce electricity. Solid-state accelerometers and gyroscopes, in addition to providing inertial tracking, also enable Digital Angel products to sense posture and gait of the wearer. This ability is important, for example, to users who are at risk for falling. Digital Angel incorporates other sensors in products depending on the desired function. Sensors currently available measure: body temperature, ambient temperature, pulse, blood pressure, sound, vibration, parts proximity, and pressure. EKG and EEG sensors will be available shortly. Inertial tracking technology - Sudden Fall Sensor - enables Digital Angel systems to sense the current posture of the wearer as well as his or her gait. This is of utmost importance to users who are at risk for falling."
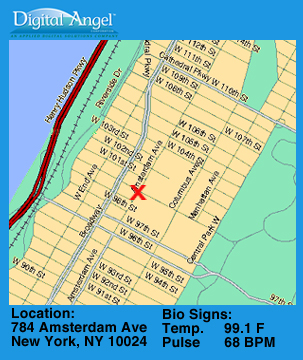
Present Use of the VeriChip™ In South America and Europe, the VeriChip produced by ADSXE is being marketed to identify kidnapped children or adults. Colombia has 3,000 kidnappings per year, the kidnap capital of the world. The
problem of kidnappings continues to get worse and worse. For example, the
city of São Paulo, Brazil home to 17 million, had 251 kidnappings in
2001 compared to 39 in 2000, and 13 in 1999.
Section 11 Other Links: http://www.sundayherald.com/print21807 http://www.cnn.com/2002/HEALTH/01/22/microchip.heart/index.html http://www.animal-id.com.au/mchips.html http://news.zdnet.co.uk/story/0,,s2083914,00.html http://www.medtronic.com/brady/clinician/medtronicpacing/clinmedt.html http://www.cnn.com/HEALTH/9908/25/brain.pacemaker/ http://news.bbc.co.uk/hi/english/health/newsid_1418000/1418091.stm
Other companies will follow Digital Angel's steps. The chips will become accepted worldwide. The technology, as the reader can see, is already developed. All it needs is public acceptance. We Christians know that the return of Jesus/Yeshua our Messiah for the rapture/rescue must be really close. May we all turn to God in our hour of trial and pray for His rescue from the things that are about to develop on this earth. Do not forget to read Time for the Rescue and may God bless you all with the spirit of understanding. | |||||||||||||||||||||||||||
 The
mark is a microchip assembly which will be implanted under
the skin of the right hand. Later on, the mark will be implanted under
the forehead, so people who have no right hand could also have the
mark. The microchip
assembly, called radio frequency identification (RFID) is already
used in animals. In dogs, the RFID is placed between
the shoulder blades, and in birds it is implanted under the wing.
Now there is one for humans called
The
mark is a microchip assembly which will be implanted under
the skin of the right hand. Later on, the mark will be implanted under
the forehead, so people who have no right hand could also have the
mark. The microchip
assembly, called radio frequency identification (RFID) is already
used in animals. In dogs, the RFID is placed between
the shoulder blades, and in birds it is implanted under the wing.
Now there is one for humans called 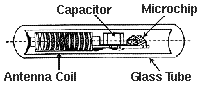 The
RFID implant/VeriChip consists of a microchip, an antenna coil, and
a capacitor all enclosed within a sealed glass tube.
The
RFID implant/VeriChip consists of a microchip, an antenna coil, and
a capacitor all enclosed within a sealed glass tube.
 When
a scanner is passed over the chip, it sends out low level radio waves
which penetrate the inert glass seal and strike the antenna. This
creates a small amount of electric current which is stored by the
capacitor. The capacitor sends the current to the microchip, which
uses the current to access its stored alpha- numeric code. The
microchip then sends the code to the antenna, where it will be picked
up and read by the scanner. An LCD on the scanning device will then
show the ID code. When
the number is displayed by the scanner it will be transmitted to an
FDA compliant secure data storage site by authorized personnel via
telephone or Internet (if used in the USA).
When
a scanner is passed over the chip, it sends out low level radio waves
which penetrate the inert glass seal and strike the antenna. This
creates a small amount of electric current which is stored by the
capacitor. The capacitor sends the current to the microchip, which
uses the current to access its stored alpha- numeric code. The
microchip then sends the code to the antenna, where it will be picked
up and read by the scanner. An LCD on the scanning device will then
show the ID code. When
the number is displayed by the scanner it will be transmitted to an
FDA compliant secure data storage site by authorized personnel via
telephone or Internet (if used in the USA).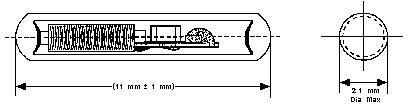
 .
.



 The
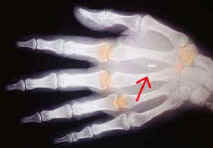
RED arrow
is pointing to the implant which we believe will be inserted under the skin of the palm by a
syringe device specially made for this type of implantation.
The
RED arrow
is pointing to the implant which we believe will be inserted under the skin of the palm by a
syringe device specially made for this type of implantation.